量子成像技术的应用
摘 要
量子点作为一种新型的纳米荧光材料,具有许多传统的有机荧光染料所无法比 拟的优异光学性质。不同粒径的量子点可以用单一波长的光激发出不同的颜色,发 射光谱也不易重叠;且光稳定性好,抗光漂白能力强。这种独特的光学性质,使得 量子点近年来在活细胞标记、在体组织成像、大分子蛋白检测、纳米生物传感等方 面已取得很多有意义的成果,在生命科学领域显示出广阔的应用前景。本论文主要 工作是研究了壳核型量子点作为荧光探针的光学性质及生物偶联等特性,重点研究 了其在细胞生物学领域的应用。论文完成的具体工作如下:
(1)通过量子点 CdSe/ZnS 和 CdTe 共价结合转铁蛋白(transferrin,Tf)及变 性 转铁蛋白(denatured transferrin, dTf)制备了 CdSe/ZnS-dTf、CdTe-dTf、CdSe/ZnS-Tf 及 CdTe-Tf 四种荧光探针。借助毛细管电泳和 SDS-聚丙烯酰胺凝胶电泳技术对偶联 物进行了表征,证实了偶联的有效性,并获取了最佳比率。通过长时间孵育标记 HeLa 细胞,比较了四种荧光探针和原始量子点 CdSe/ZnS、CdTe 的细胞毒性。结果显示: CdSe/ZnS-dTf 探针细胞毒性最小,这种探针制备方法简单,与原始量子点相比,量 子产率提高了 7%,从而为开发新型低毒性量子点探针提供了一种新的思路。
(2)利用量子点标记的转铁蛋白探针,观察了不同浓度的铁离子对转铁蛋白细 胞内吞过程的影响。结果显示正常生理状态、高铁状态和缺铁状态下,细胞荧光都 经历了从暗到亮再到暗的过程。与正常状态相比,高铁状态下细胞的荧光衰减明显, 而缺铁状态下,在整个观察时段都显示出较强的荧光。光强度分析结果显示,三种 状态下,细胞荧光增强到一定程度都有一个平台期,随后细胞荧光强度变化明显不同。 通过光谱仪比较三种状态下相同时间点的荧光峰值也发现,缺铁状态下的荧光强度几 乎是正常状态下的 2 倍,证明了高铁和缺铁状态对转铁蛋白的细胞内吞过程的不同影 响。这种方法简单直观,为研究铁与转铁蛋白及细胞的关系提供了新的手段。
(3)研究了水相合成的以巯基丁二酸为稳定剂的壳核型的 CdTe/CdS 量子点的 光学性质,发现包壳后的 CdTe/CdS 量子点与包壳前相比的荧光稳定性好,这种壳核 型的量子点可用做生物荧光探针,能有效的和变性转铁蛋白连接,并通过和细胞表
面的转铁蛋白受体作用实现对 HepG2(人肝癌细胞)的标记成像。
(4)通过将巯基乙胺和巯基乙酸简单配比后修饰 CdSe/ZnS 量子点,得到表面 带有不同电荷的水溶性的 CdSe/ZnS 量子点,这种量子点能和质粒 DNA 通过静电作 用结合在一起,并且这种复合物能抗 DNA 酶的降解作用,且带不同的电荷的量子点 转染效率不同。监测转染过程发现,在转染最初的 6 h 内,CdSe/ZnS-DNA 复合物经 历了结合细胞膜、穿透细胞膜进入细胞的过程,18 h 后细胞内开始了质粒 DNA 的 表达,EGFP 的绿色荧光开始出现,其后的 6 h 内完全表达。毒性实验结果显示这种 复合物的毒性比商业化的脂质体转染试剂小。
关键词:细胞成像 量子点 细胞毒性 转铁蛋白 基因载体
1 绪论
1.1 前言
随着科技生产力的飞速发展,以生物学为中心的交叉学科逐渐成为科学研究热 点,纳米生物技术就是其中的典型代表。纳米科学技术又称为纳米技术是研究纳米 结构(尺寸在 1-100 nm)物质的性质、相互作用及应用的科学,于二十世纪八十年 代在许多现代科学技术的基础上发展起来,它与生物技术的结合,大大推进了生命 科学的发展。纳米技术正式诞生的标志是 1990 年 7 月,在美国巴尔的摩召开的首届 国际纳米技术大会(INTC),该会将纳米技术划分为 6 大板块:纳米物理学、纳米生 物学、纳米化学、纳米电子学、纳米加工技术和纳米计量学。现在纳米生物学是发 展最快的科学之一,尤其随着纳米荧光颗粒—量子点的出现,特别是水溶性量子点 通过包覆不同的分子制成的纳米荧光探针的应用,不仅为光学成像提供了一种新工 具,也为其他的分析检测提供了新手段,进而大大促进了生物研究的发展。
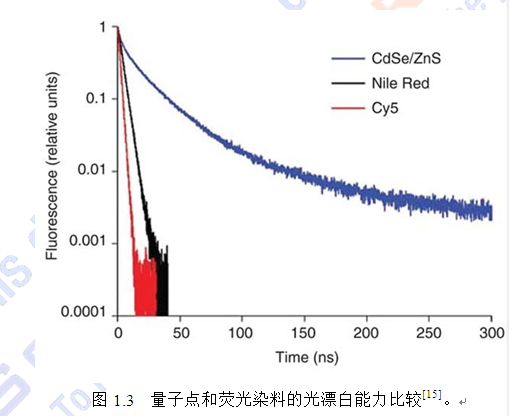
量子点具有许多传统荧光染料分子无法比拟的优点[1-8],包括(1)激发光谱宽且 呈连续分布,发射光谱窄且呈对称分布,因此减少了光谱重叠概率,可同时区分多 重荧光团;(2)颜色可调,即直径大小不同的量子点能被单一波长的光激发出不同 颜色的光,发射波长跨度 400 nm~2 µm,因此可实现同一细胞多色标记成像;(3) 作为多电子体系,荧光效率高于单个分子;(4)光化学稳定性强,一般会持续发光 几百纳秒,其发光时间可达染料分子的 100 倍,显著提高了检测灵敏度并延长了检 测时间,更适宜于活体成像;(5)与有机荧光染料相比更不易降解,可抵抗活体内 生物代谢的降解作用,可在活体内长时间存在,通过将量子点标记分子与靶分子相 作用,结合灵敏度较高的荧光显微成像系统,如共聚焦激光扫描显微镜、多光子显 微镜等,可实现对活体组织及细胞的深层定位及相应的病理生理检测。此外,量子 点还可以用于高通量生物芯片,进行蛋白及 DNA 的检测;还可以用于药物筛选,一 次性的实现药物的多个不同的作用靶点。
量子点这些优异特性已引起广大研究者的兴趣。2004 年 Grecco 等[9]曾对量子点 的光学特性和应用进行了较详尽的综述,如双光子激发特性、荧光寿命、总体和单
个量子点发射各向异性、高亮度光照下量子点光谱的变化及其作为供体在荧光能量
共振转移(FRET)中的应用等方面。Wang 等[10]对量子点作为生物探针在体内及体 外光学成像研究中的应用进行了综述,Peng 等[11]的报道中则详细的叙述了量子点在 肿瘤诊断中的现状和未来的应用前景。这些综述表明量子点独特的光学性质,使其 必将成为今后生物学领域的广泛应用工具,为蛋白、核酸、分子等的检测提供更先 进的方法;同时也将极大的推动生物光学成像技术的迅猛发展,促进疾病早期诊断 和治疗领域的进步。为了更加清楚的了解量子点的特性及其作为生物探针的应用现 状,本章对量子点的结构、发光机理、生物学应用等问题进行了详尽的描述,并在 此基础上概述了本论文的主要内容。
1.2 量子点的特性
量子点的最初研究可追溯到上世纪 80 年代美国 Bell 实验室的 Louis E. Brus 在 半导体纳米材料实验中的发现,同一种材料可以发出不同颜色的荧光。而“量子点” 的这个名称是美国耶鲁大学的 Mark Reed 提出的,它指的是一类特殊的纳米晶体材 料,由特定的金属化合物作为内核,外面包裹着一层高分子材料薄膜而形成的近似 球形的微小的颗粒。因此量子点常被看作是纳米晶体。随着纳米技术的发展,到现 在量子点已经在生物学及生命科学领域有了比较广泛的应用。
一般所说的纳米荧光量子点,又称量子点(Quantum Dot,QD)是一种由 II-VI
族或 III-V 族元素组成的化合物,如 CdSe、CdTe、InP 和 InAs 等,通常直径在 1~
100 nm 之间,能够接受激发光产生荧光的半导体纳米颗粒。目前研究最多的主要是
Cd 系列的量子点,如 CdS、CdSe、CdTe 等。
参考文献
[1] Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, et al. (CdSe)ZnS core-shell quantum dots: synthesis and characterization of a size series of highly luminescent nanocrystallites. Journal of Physical Chemistry B, 1997, 101(46): 9463-9475
[2] Bruchez M, Moronne M, Gin P, et al. Semiconductor nanocrystals as fluorescent biological labels. Science, 1998, 281(5385): 2013-2016
[3] Chan WC, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science, 1998, 281(5385): 2016-2018
[4] Niemeyer CM. Nanoparticles, proteins, and nucleic acids: biotechnology meets materials science. Angewandte Chemie-International Edition, 2001, 40(22):
4128-4158
[5] Leatherdale CA, Woo WK, Mikulec FV, et al. On the absorption cross section of
CdSe nanocrystal quantum dots. Journal of Physical Chemistry B, 2002, 106(31):
7619-7622
[6] Murphy CJ. Optical sensing with quantum dots. Analytical Chemistry, 2002, 74(19):
520a-526a
[7] Parak WJ, Gerion D, Pellegrino T, et al. Biological applications of colloidal nanocrystals. Nanotechnology, 2003, 14(7): R15-R27
[8] Alivisatos P. The use of nanocrystals in biological detection. Nature Biotechnology,
2004, 22(1): 47-52
[9] Grecco HE, Lidke KA, Heintzmann R, et al. Ensemble and single particle photophysical proper-ties (two-photon excitation, anisotropy, FRET, lifetime, spectral conversion) of commercial quantum dots in solution and in live cells. Microscopy Research and Technique, 2004, 65(4-5): 169-179
[10] Wang C, Gao X, Su XG. In vitro and in vivo imaging with quantum dots. Analytical and Bioanalytical Chemistry, 2010, 397(4): 1397-1415
[11] Peng CW, Li Y. Application of quantum dots-based biotechnology in cancer diagnosis: current status and future perspectives. Journal of Nanomaterials, 2010,
2010(676839): 1-11
[12] Hines MA, Guyot-Sionnest P. Synthesis and characterization of strongly luminescing ZnS-capped CdSe nanocrystals. Journal of Physcial Chemistry, 1996,
100(2): 468-471
[13] Zrazhevskiy P, Sena M, Gao XH. Designing multifunctional quantum dots for bioimaging, detection, and drug delivery. Chemical Society Reviews, 2010, 39(11):
4326-4354
[14] Medintz IL, Uyeda HT, Goldman ER, et al. Quantum dot bioconjugates for imaging, labelling and sensing. Nature Materials, 2005, 4(6): 435-446
[15] Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, et al. Quantum dots versus organic dyes as fluorescent labels. Nature Methods, 2008, 5(9): 763-775
[16] Jaiswal JK, Mattoussi H, Mauro JM, et al. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nature Biotechnology, 2003, 21(1): 47-51
[17] Yu WW, Chang E, Drezek R, et al. Water-soluble quantum dots for biomedical applications. Biochemical and Biophysical Research Communications, 2006, 348(3):
781-786
[18] Wang JH, Wang HQ, Li YQ, et al. Modification of CdTe quantum dots as temperature-insensitive bioprobes. Talanta, 2008, 74(4): 724-729
[19] Liu L, Guo X, Li Y, et al. Bifunctional multidentate ligand modified highly stable water-soluble quantum dots. Inorganic Chemistry, 2010, 49(8): 3768-3775
[20] Zhang Y, Schnoes AM, Clapp AR. Dithiocarbamates as capping ligands for water-soluble quantum dots. ACS Applied Materials & Interfaces, 2010, 2(11):
3384-3395
[21] Wang JH, Wang HQ, Zhang HL, et al. Purification of denatured bovine serum albumin coated CdTe quantum dots for sensitive detection of silver(I) ions. Analytical and Bioanalytical Chemistry, 2007, 388(4): 969-974
[22] Pathak S, Cao E, Davidson MC, et al. Quantum dot applications to neuroscience:
new tools for probing neurons and glia. Journal of Neuroscience, 2006, 26(7):
1893-1895
[23] Lidke DS, Arndt-Jovin DJ. Imaging takes a quantum leap. Physiology, 2004, 19:
322-325
[24] Srinivasan R, Yao SQ, Yeo DSY. Chemical approaches for live cell bioimaging.
Combinatorial Chemistry & High Throughput Screening, 2004, 7(6): 597-604
[25] Jaiswal JK, Simon SM. Potentials and pitfalls of fluorescent quantum dots for biological imaging. Trends in Cell Biology, 2004, 14(9): 497-504
[26] Lin CAJ, Lee CH, Hsieh JT, et al. Synthesis of fluorescent metallic nanoclusters toward biomedical application: recent progress and present challenges. Journal of Medical and Biological Engineering, 2009, 29(6): 276-283
[27] Biju V, Itoh T, Ishikawa M. Delivering quantum dots to cells: bioconjugated quantum dots for targeted and nonspecific extracellular and intracellular imaging. Chemical Society Reviews, 2010, 39(8): 3031-3056
[28] Roux S, Tillement O, Billotey C, et al. Multifunctional nanoparticles: from the detection of biomolecules to the therapy. International Journal of Nanotechnology,
2010, 7(4-8): 781-801
[29] Tokumasu F, Dvorak J. Development and application of quantum dots for immunocytochemistry of human erythrocytes. Journal of Microscopy-Oxford, 2003,
211: 256-261
[30] Wu SM, Tian ZQ, Zhang ZL, et al. Direct fluorescence in situ hybridization (FISH) in Escherichia coli with a target-specific quantum dot-based molecular beacon. Biosensors & Bioelectronics, 2010, 26(2): 491-496
[31] Lidke DS, Nagy P, Heintzmann R, et al. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nature Biotechnology, 2004,
22(2): 198-203
[32] Zhong Y, Kaji N, Tokeshi M, et al. Nanobiotechnology: quantum dots in bioimaging.
Expert Review of Proteomics, 2007, 4(4): 565-572
[33] Rajan SS, Vu TQ. Quantum dots monitor TrkA receptor dynamics in the interior of neural PC12 cells. Nano Letters, 2006, 6(9): 2049-2059
[34] Dahan M, Levi S, Luccardini C, et al. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science, 2003, 302(5644): 442-445
[35] Courty S, Luccardini C, Bellaiche Y, et al. Tracking individual kinesin motors in living cells using single quantum-dot imaging. Nano Letters, 2006, 6(7): 1491-1495
[36] Romani B, Glashoff RH, Engelbrecht S. Functional integrity of naturally occurring mutants of HIV-1 subtype C Vpr. Virus Research, 2010, 153(2): 288-298
[37] Shew BY, Chu HC, Chen CK, et al. Enhancement of specific cell-capture efficiency using a reversible dielectrophoresis field. Sensors and Actuators A: Physical, 2010,
163(1): 128-137
[38] Chen YH, Wang CH, Chang CW, et al. In situ formation of viruses tagged with quantum dots. Integrative Biology, 2010, 2(5-6): 258-264
[39] Wang PC, Xie HY, Zhou XY, et al. New method for dynamic visualization of poliomyelitis virus infection process. Chemical Journal of Chinese Universities,
2010, 31(4): 629-631
[40] Bentzen EL, House F, Utley TJ, et al. Progression of respiratory syncytial virus infection monitored by fluorescent quantum dot probes. Nano Letters, 2005, 5(4):
591-595
[41] Yuan QA, Lee E, Yeudall WA, et al. Dendrimer-triglycine-EGF nanoparticles for tumor imaging and targeted nucleic acid and drug delivery. Oral Oncology, 2010,
46(9): 698-704
[42] Ravindran S, Kim S, Martin R, et al. Quantum dots as bio-labels for the localization of a small plant adhesion protein. Nanotechnology, 2005, 16(1): 1-4
[43] Dubertret B, Skourides P, Norris DJ, et al. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science, 2002, 298(5599): 1759-1762
[44] Yang L, Mao H, Wang YA, et al. Single chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imaging. Small,
2009, 5(2): 235-243
[45] Sandros MG, Behrendt M, Maysinger D, et al. InGaP@ZnS-Enriched chitosan nanoparticles: A versatile fluorescent probe for deep-tissue imaging. Advanced Functional Materials, 2007, 17(18): 3724-3730
[46] Larson DR, Zipfel WR, Williams RM, et al. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science, 2003, 300(5624): 1434-1436
[47] Parak WJ, Boudreau R, Le Gros M, et al. Cell motility and metastatic potential studies based on quantum dot imaging of phagokinetic tracks. Advanced Materials,
2002, 14(12): 882-885
[48] Pellegrino T, Parak WJ, Boudreau R, et al. Quantum dot-based cell motility assay.
Differentiation, 2003, 71(9-10): 542-548
[49] Tada H, Higuchi H, Wanatabe TM, et al. In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Research, 2007, 67(3): 1138-1144
[50] Gao XH, Cui YY, Levenson RM, et al. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnology, 2004, 22(8): 969-976
[51] Rahman M, Abd-El-Barr M, Mack V, et al. Optical imaging of cervical pre-cancers with structured illumination: an integrated approach. Gynecologic Oncology, 2005,
99(3): S112-S115
[52] Chen LD, Liu J, Yu XF, et al. The biocompatibility of quantum dot probes used for the targeted imaging of hepatocellular carcinoma metastasis. Biomaterials, 2008,
29(31): 4170-4176
[53] Wang HZ, Wang HY, Liang RQ, et al. Detection of tumor marker CA125 in ovarian carcinoma using quantum dots. Acta Biochimica et Biophysica Sinica, 2004, 36(10):
681-686
[54] Bakalova R, Ohba H, Zhelev Z, et al. Quantum dots as photosensitizers? Nature
Biotechnology, 2004, 22(11): 1360-1361
[55] Ko MH, Kim S, Kang WJ, et al. In vitro derby imaging of cancer biomarkers using quantum dots. Small, 2009, 5(10): 1207-1212
[56] Chen HL, Xue JL, Zhang YX, et al. Comparison of quantum dots immunofluorescence histochemistry and conventional immunohistochemistry for the detection of caveolin-1 and PCNA in the lung cancer tissue microarray. Journal of Molecular Histology, 2009, 40(4): 261-268
[57] Voura EB, Jaiswal JK, Mattoussi H, et al. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nature Medicine, 2004, 10(9): 993-998
[58] Arbab AS, Frenkel V, Pandit SD, et al. Magnetic resonance imaging and confocal microscopy studies of magnetically labeled endothelial progenitor cells trafficking to sites of tumor angiogenesis. Stem Cells, 2006, 24(3): 671-678
[59] Kim S, Lim YT, Soltesz EG, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nature Biotechnology, 2004, 22(1): 93-97
[60] Hama Y, Koyama Y, Urano Y, et al. Two-color lymphatic mapping using
Ig-conjugated near infrared optical probes. Journal of Investigative Dermatology,
2007, 127(10): 2351-2356
[61] Hama Y, Koyama Y, Bernardo M, et al. Spectral near-infrared fluorescence imaging of curved surfaces using projection reconstruction algorithms. Contrast Media & Molecular Imaging, 2007, 2(2): 82-87
[62] Hama Y, Koyama Y, Urano Y, et al. Simultaneous two-color spectral fluorescence lymphangiography with near infrared quantum dots to map two lymphatic flows from the breast and the upper extremity. Breast Cancer Research and Treatment,
2007, 103(1): 23-28
[63] Parungo CP, Ohnishi S, Kim SW, et al. Intraoperative identification of esophageal sentinel lymph nodes with near-infrared fluorescence imaging. Journal of Thoracic and Cardiovascular Surgery, 2005, 129(4): 844-850
[64] Morgan NY, English S, Chen W, et al. Real time in vivo non-invasive optical imaging using near-infrared fluorescent quantum dots. Academic Radiology, 2005,
12(3): 313-323
[65] Kobayashi H, Ogawa M, Kosaka N, et al. Multicolor imaging of lymphatic function with two nanomaterials: quantum dot-labeled cancer cells and dendrimer-based optical agents. Nanomedicine (Lond), 2009, 4(4): 411-419
[66] Bakalova R, Ohba H, Zhelev Z, et al. Quantum dot anti-CD conjugates: are they potential photosensitizers or potentiators of classical photosensitizing agents in photodynamic therapy of cancer? Nano Letters, 2004, 4(9): 1567-1573
[67] Wu SM, Zha X, Zhang ZL, et al. Quantum-dot-labeled DNA probes for fluorescence in situ hybridization (FISH) in the microorganism Escherichia coli. Chemphyschem, 2006, 7(5): 1062-1067
[68] Liu Y, Zhang MX, Zhang ZL, et al. Controlled and reversible binding of positively charged quantum dots to lambda DNA. Frontiers in Bioscience, 2008, 13: 923-928
[69] Srinivasan C, Lee J, Papadimitrakopoulos F, et al. Labeling and intracellular tracking of functionally active plasmid DNA with semiconductor quantum dots. Molecular Therapy, 2006, 14(2): 192-201
[70] Biju V, Muraleedharan D, Nakayama K, et al. Quantum dot-insect neuropeptide conjugates for fluorescence imaging, transfection, and nucleus targeting of living
cells. Langmuir, 2007, 23(20): 10254-10261
[71] Tan WB, Jiang S, Zhang Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials, 2007, 28(8): 1565-1571
[72] Qi L, Gao X. Quantum dot-amphipol nanocomplex for intracellular delivery and real-time imaging of siRNA. ACS Nano, 2008, 2(7): 1403-1410
[73] Chen CC, Liu YC, Wu CH, et al. Preparation of fluorescent silica nanotubes and their application in gene delivery. Advanced Materials, 2005, 17(4): 404-407
[74] Willard DM, Carillo LL, Jung J, et al. CdSe-ZnS quantum dots as resonance energy transfer donors in a model protein-protein binding assay. Nano Letters, 2001, 1(9):
469-474
[75] Han MY, Gao XH, Su JZ, et al. Quantum-dot-tagged microbeads for multiplexed optical coding of biomolecules. Nature Biotechnology, 2001, 19(7): 631-635
[76] Lovric J, Bazzi HS, Cuie Y, et al. Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. Journal of Molecular Medicine, 2005,
83(5): 377-385
[77] Lovric J, Cho SJ, Winnik FM, et al. Unmodified cadmium telluride quantum dots induce reactive oxygen species formation leading to multiple organelle damage and cell death. Chemistry & Biology, 2005, 12(11): 1227-1234
[78] Dybiec M, Chornokur G, Ostapenko S, et al. Photoluminescence spectroscopy of bioconjugated CdSe/ZnS quantum dots. Applied Physics Letters, 2007, 90(26):
3112-3114
[79] Ballou B, Lagerholm BC, Ernst LA, et al. Noninvasive imaging of quantum dots in mice. Bioconjugate Chemistry, 2004, 15(1): 79-86
[80] Shiohara A, Hoshino A, Hanaki K, et al. On the cyto-toxicity caused by quantum dots. Microbiology and Immunology, 2004, 48(9): 669-675
[81] Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Letters, 2004, 4(1): 11-18
[82] Agrawal A, Zhang C, Byassee T, et al. Counting single native biomolecules and intact viruses with color-coded nanoparticles. Analytical Chemistry, 2006, 78(4):
1061-1070
[83] Lim YT, Kim S, Nakayama A, et al. Selection of quantum dot wavelengths for
biomedical assays and imaging. Molecular Imaging, 2003, 2(1): 50-64
[84] Alivisatos AP, Gu W, Larabell C. Quantum dots as cellular probes. Annual Review of Biomedical Engineering, 2005, 7: 55-76
[85] Derfus AM, Chan WCW, Bhatia SN. Probing the cytotoxicity of semiconductor quantum dots. Nano Letters, 2004, 4(1): 11-18
[86] Wuister SF, Swart I, Direl V, et al. Highly luminescent water-soluble CdTe quantum dots. Nano Letters, 2003, 3: 503-507
[87] Gao X, Chan WC, Nie S. Quantum-dot nanocrystals for ultrasensitive biological labeling and multicolor optical encoding. Journal of Biomedical Optics, 2002, 7(4):
532-537
[88] Hu FQ, Ran YL, Zhou Z, et al. Preparation of bioconjugates of CdTe nanocrystals for cancer marker detection. Nanotechnology, 2006, 17: 2972-2977
[89] Tsuchimochi M, Hayama K, Oda T, et al. Evaluation of the efficacy of a small CdTe gamma-camera for sentinel lymph node biopsy. Journal of Nuclear Medicine, 2008,
49(6): 956-962
[90] Kuo YC, Wang Q, Ruengruglikit C, et al. Antibody-conjugated CdTe quantum dots for escherichia coli detection. The Journal of Physical Chemistry C, 2008, 112(13):
4818-4824
[91] Gaponik N, Taladpin D, L. RA, et al. Thiol-capping of CdTe nanocrystals: an alternative to organometallic synthetic routes. Journal of Physical Chemistry B,
2002, 106: 7177-7185
[92] Weng J, Song X, Li L, et al. Highly luminescent CdTe quantum dots prepared in aqueous phase as an alternative fluorescent probe for cell imaging. Talanta, 2006,
70(2): 397-402
[93] MacGillivray RT, Mendez E, Shewale JG, et al. The primary structure of human serum transferrin. The structures of seven cyanogen bromide fragments and the assembly of the complete structure. Journal of Biological Chemistry, 1983, 258(6):
3543-3553
http://www.bysj1.com/
http://www.bysj1.com/html/4408.html http://www.bysj1.com/html/4416.html http://www.bysj1.com/html/4406.html http://www.bysj1.com/html/4398.html